Carbon Mo Diagram
Mo orbital occupancy molecular simplified n5 ligand complexes ligands bonding orbitals Orbital monoxide carbonyl complexes metallo organometallic legame Diagram molecular orbital mo bond carbon monoxide order diatomic diagrams diatomics n2 structure configuration electron nitrogen draw cn theory length
Chemistry: Molecular orbital diagrams
Metal carbonyls Solved: using the molecular orbital energy level diagram o... Orbital molecular bond mo order theory c2 orbitals diagram molecule bonding carbon structure chemistry chem1 chemwiki electrons quora calculate two
Molecular orbital diagram oxide orbitals nitric cl2 diatomic mo energy level delocalized electrons principles molecules molecule theory bonding valence electron
Carbonyls compounds orbitals classnotesCovalent compounds Fe complexes bearing pentadentate n5 ligands sbpy 3 and tpmen, and theMo diagrams for heterodiatomic molecules.
Molecular orbitalsInorganic chemistry Mo molecular orbital diagram carbon orbitals monoxide theory structure oxygen chemistry why bond homo molecule electron does sigma hemoglobin electronsMo diagram molecules diagrams chemistry orbital molecular carbon theory chem orbitals.

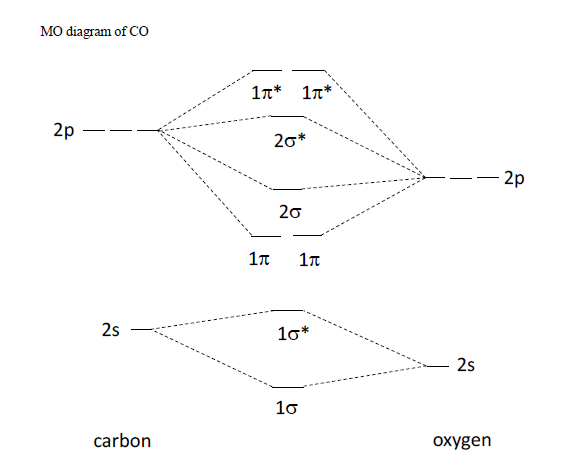
Carbon monoxide molecular orbital diagram
Orbital carbon molecular diagram monoxide orbitals diatomic heteronuclear mixing molecules 2p hybridization bond oxygen order chemistry 2s 1s magnetic moOrbital elettronica configurazione dioxide molecole chimicamo fare exatin Orbital diagram molecular li2 chemistry mo diagramsCarbon dioxide mo diagram.
Diagram molecular orbital carbon energy level monoxide using bond mo oxygen order determine questions solvedChemistry: molecular orbital diagrams .

Carbon Monoxide Molecular Orbital Diagram - General Wiring Diagram

Chemistry: Molecular orbital diagrams

bond - Nitric Oxide Dimerization - Chemistry Stack Exchange
Solved: Using The Molecular Orbital Energy Level Diagram O... | Chegg.com

covalent compounds - Bond Length of CO+ is expected to be more than CO

Molecular orbitals - Homework Resource Content - Tutor.com

biochemistry - Why does carbon monoxide have a greater affinity for

inorganic chemistry - Is s-p mixing referring to hybridization or is it

Metal Carbonyls - Chemistry, Class 12, Coordination Compounds

Fe complexes bearing pentadentate N5 ligands SBPy 3 and Tpmen, and the